Each year, approximately 2.2 million bone graft surgeries are performed globally, yet the repair of large-volume bone defects remains a significant clinical challenge. Currently, bone repair materials are mainly categorized into three types: autologous bone, natural bone grafts, and synthetic bone substitutes. Among them, autologous bone grafting is limited by donor shortages and secondary trauma, while allografts and metal implants face issues such as immune rejection and mechanical mismatch.
Biodegradable polyester materials (e.g., PLA, PLGA, PCL) can be precisely fabricated into biomimetic porous scaffolds using advanced technologies such as 3D printing. With their controllable degradation characteristics, excellent biocompatibility, and tunable mechanical properties, these materials offer a breakthrough solution for bone regenerationâparticularly in the repair of critical-sized defects following trauma or tumor resection.
Key Advantages of Biodegradable Polyesters:
â Excellent biodegradability and clear in vivo metabolic pathways;
â Outstanding processability, especially compatible with 3D printing and custom design;
â Adjustable degradation cycles that can be well-synchronized with bone healing processes.
However, to enhance the mechanical strength and osteoinductive activity of polyester scaffolds, researchers often combine them with inorganic ceramics such as hydroxyapatite (HAp) and tricalcium phosphate (TCP). In particular, nano-hydroxyapatite (nHAp), which mimics the mineral components of natural bone, exhibits strong osteoinductive potential and drug release capacity. In complex models such as maxillofacial bone defects, polyester and composite scaffolds have demonstrated excellent bone integration and mechanical support capabilities.
So, how can we optimize structure and integrate functionality to unleash the full potential of biodegradable polyesters in bone repair?
I. From Structure to Function: Core Logic in Scaffold Design
Bone regeneration is more than spatial fillingâit is a multi-dimensional coordinated system involving structural mechanics, cellular behavior, and the tissue microenvironment. The design of polyester scaffolds is transitioning from static âmimicryâ to dynamic âfunctionality,â focusing on three key aspects:
1. Microstructure Design: Pore Size, Porosity, and Connectivity
Pore architecture forms the foundation of scaffold bioactivity.
â Optimal pore size range: 300â500 μm, which promotes bone ingrowth, vascularization, and nutrient transport;
â Recommended porosity: 70â90%. Excessive porosity may compromise mechanical strength;
â Gradient structure design: Load-bearing areas (e.g., outer mandible) benefit from smaller pores for support, while low-load areas (e.g., sinus floor) can adopt larger pores to enhance cell infiltration.
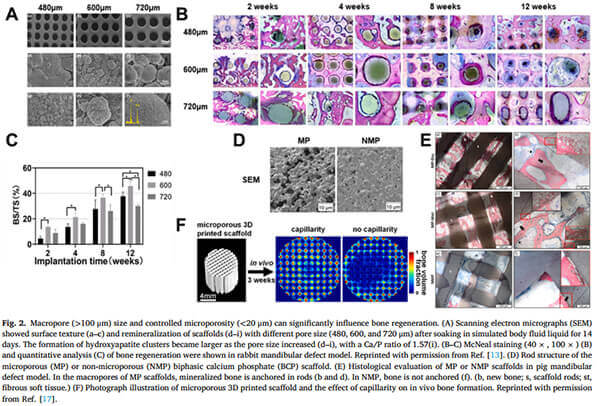
Studies show that large pore diameters (600 μm or even 1000 μm) can lead to improved bone formation in specific anatomical sites.
(A) Scanning Electron Microscope (SEM) images show surface textures and remineralization of scaffolds with different pore sizes; larger pores correspond to increased hydroxyapatite cluster formation.
(B-C) McNeal staining (40Ã, 100Ã) and quantitative analysis in a rabbit mandibular defect model indicate enhanced bone regeneration.
(D)Micro-porous (MP) vs. non-micro-porous (NMP) biphasic calcium phosphate (BCP) scaffolds.
(E) Histological evaluation in a porcine mandibular defect model reveals that mineralized bone anchors within MP scaffolds (B and d), but not in NMP scaffolds (F), emphasizing the role of capillarity in promoting in vivo bone formation [1].
2.Degradation Kinetics Coordinated with Bone Healing
The degradation rate of the material should match the rhythm of tissue regeneration to achieve âtimely disappearanceâ:
- PLGA degradation can be tuned by adjusting the lactic acid/glycolic acid ratio;
- Ceramic components such as TCP release Ca²âº/POâ³⻠upon degradation, directly activating osteogenic pathways;
- Rapid degradation may cause structural collapse, while slow degradation could hinder new bone integration.
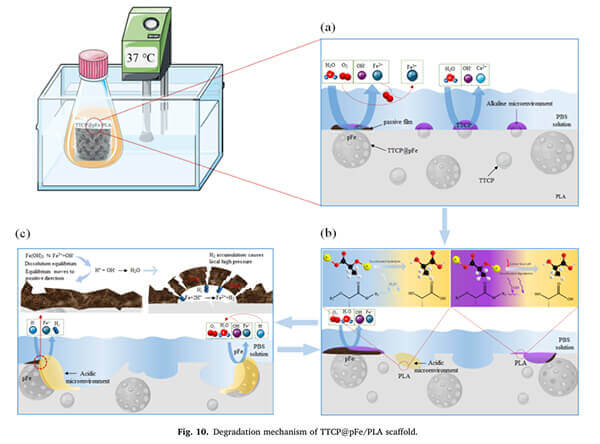
Studies have shown that PLA scaffolds combined with tetracalcium phosphate (TTCP) and porous iron (pFe) (i.e., TTCP@pFe/PLA) generate an alkaline microenvironment via TTCP dissolution, which effectively catalyzes PLA hydrolysis and accelerates degradation [2].
3. Mechanical Adaptability
Proper mechanical matching not only enhances early stability but also promotes osteogenesis:
- The elastic modulus of polyester composite scaffolds should match the bone density at different anatomical sites;
- In high-shear zones (e.g., mandibular masticatory regions), a balance between rigidity and toughness is essential;
- Porous design must simultaneously address mechanical strength and functional channel configuration.
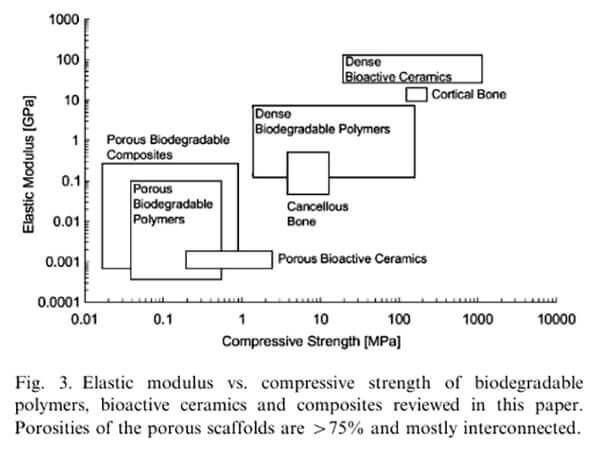
Generally, scaffold compressive modulus should fall between that of trabecular bone (0.01â2.0 GPa) and cortical bone (8â25 GPa). Scaffold performance can be adjusted accordingly to the required compressive strength at different implantation sites. Some dense polymer scaffolds can achieve mechanical properties comparable to trabecular or even cortical bone [3].
Scaffold performance targets during fabrication [4].
II. Smart Responsiveness: Advanced Scaffold Design Logic
Researchers are currently developing intelligent scaffolds with âenvironmental sensingâ capabilities, transforming them from passive materials into active microenvironment modulators. Key directions include:
1. External Stimuli Responsiveness
External physical stimuli such as light, magnetic fields, electricity, ultrasound, and mechanical loading can precisely trigger functional modules within scaffolds, enabling:
- Photothermal response (e.g., PLGA + black phosphorus nanosheets): Near-infrared irradiation induces localized heating and upregulation of osteogenic proteins;
- Magnetothermal response (e.g., FeâOâ/PLGA composites): Magnetic fields generate localized heating to ablate bone tumors and promote osteogenesis;
- Electrical response (e.g., polypyrrole (PPy)-doped polyesters): Activates calcium signaling pathways and enhances expression of osteogenic genes;
- Ultrasound response: Low-frequency ultrasound triggers release of growth factors or antibacterial agents, suitable for deep tissue therapy.
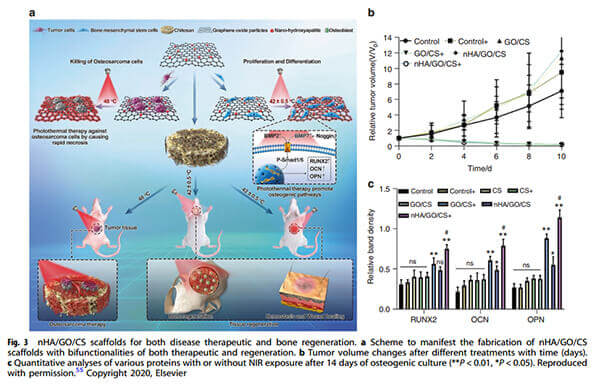
An example: nHA/GO/CS scaffolds with dual therapeutic and regenerative functions.
(a) Scaffold fabrication scheme.
(b) Tumor volume changes over time following various treatments.
(c) Quantitative analysis of protein expression after 14 days of osteogenic culture with or without NIR exposure [5].
2. Endogenous Microenvironment Responsiveness
- ROS overexpression: Triggers release of antioxidants or osteoinductive factors;
- pH-responsive systems: Detect acidification due to inflammation/infection and adjust degradation or drug release accordingly;
- Controlled release of loaded factors: Incorporates BMP-2, VEGF, or antimicrobial peptides for staged osteogenesis and infection prevention.
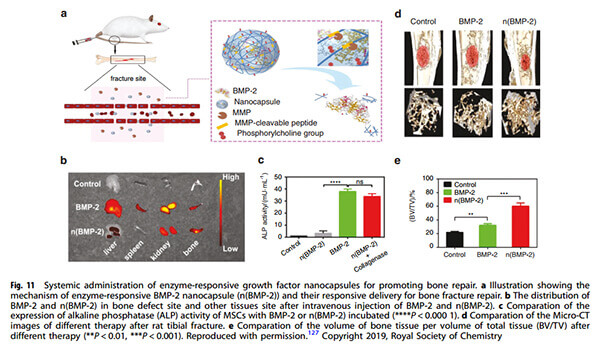
Example: Enzyme-responsive BMP-2 nanocapsules (n(BMP-2)) for fracture repair.Distribution of intravenously injected BMP-2 in bone defect and other tissues.Comparison of ALP activity in MSCs incubated with BMP-2.Micro-CT comparison of tibial fractures in rats under different treatments.Bone volume/total volume (BV/TV) ratio comparisons [5].
3. Structural Modulation
- Immunomodulatory structures: Guide macrophage polarization from M1 to M2 phenotype, achieving a synergistic âanti-inflammatory to osteogenicâ transition;
- Biomimetic multilayer scaffolds: Mimic cortical-cancellous bone architecture to enhance cell adhesion and spatial differentiation.
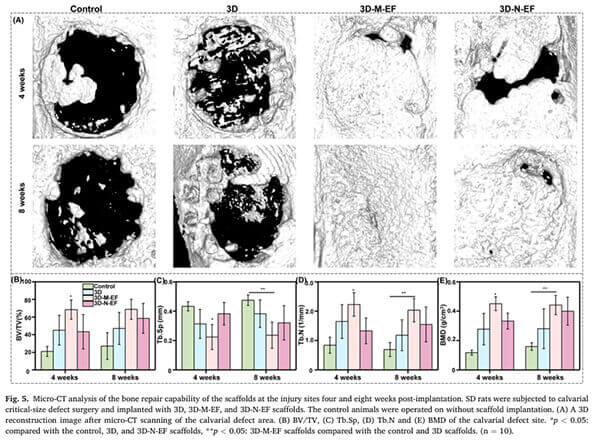
Example: Liu et al. used PLLA microfibrous scaffolds to polarize more RAW264.7 cells to M2 macrophages. In vivo studies in rat cranial defects confirmed accelerated new bone formation [6].
These composite and responsive strategies are gradually advancing from the lab to large animal models and early clinical research, representing the developmental trend of bone tissue engineering (BTE) scaffold materials. Due to their excellent processability and composite adaptability, polyesters are ideal âmatricesâ for hosting these functional modules.
III.Biodegradable Medical Polyester Material Solutions
eSUNMed specializes in medical monomers, biodegradable polyesters (including PLA, PCL, PGA, and their copolymers), polyester microspheres, polyester-based composites, FDM 3D printing filaments, and SLS 3D printing powders. It also offers high-precision fabrication services to support the development and application of implantable, resorbable medical devices.
With the introduction of 4D printing, AI-based structural modeling, and bioinformatics simulation, polyester composite scaffolds are gradually evolving into âsystematic materialsâ with integrated functions, personalized applications, and controlled manufacturing.
Conclusion: Polyester as the Foundation, Composites as the WingsâThe Future of Bone Regeneration is Malleable
Bone repair is not just a problem of materials scienceâit is a systemic regulatory challenge. Biodegradable polyesters provide the structural foundation and tunable metabolic process for bone regeneration, while their combination with bioceramics, drugs, and cytokines adds âfunctional wings.â
The next generation of polyester scaffolds will not merely be biodegradable, but truly guidable, tunable, and adaptive biomaterial systemsâpropelling us into a new era of personalized and intelligent regenerative medicine.
Recommended References:
1.Huang, Xiangya, et al. âBiomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances.âBioactive Materials, vol. 33, 2024, pp. 129-156.
2.Zhu,Shunshun,et al.”Research Progress in 3D Printed Biobased and Biodegradable Polyester/Ceramic Composite Materials: Applications and Challenges in Bone Tissue Engineering.” ACS Applied Materials & Interfaces, vol. 17, no. 1, 2025, pp. 2791-2813.
3.Rezwan, K., et al. “Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering.” Biomaterials, vol. 27, no. 18, 2006, pp. 3413-3431.
4.Percival, Kelly M, et al. âRecent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration.âInternational  Journal of Molecular Sciences, vol. 25, no. 11, 2024, p. 6012.
5.Wei, Hongpu, et al. “Recent Advances in Smart Stimuli-Responsive Biomaterials for Bone Therapeutics and Regeneration.” Bone Research, vol. 10, 2022, p. 17.
6.Liu, Xingzhi, et al. “Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration.” Biomaterials, vol. 276, 2021, p. 121037.

