On July 11, 2025, eSUNMed successfully completed the master file registration of its independently developed medical-grade PLGA raw material with the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). The registration number is (M2025250-000). This milestone signifies that this core material has received formal regulatory recognition for applications in drug delivery systems, medical devices, and related fields, laying a crucial foundation for the companyŌĆÖs future medical device registration and regulatory submissions.

Poly(lactic-co-glycolic acid) (PLGA) is a degradable biomedical polymer synthesized via random copolymerization of lactic acid and glycolic acid monomers. Its excellent biocompatibility and controllable degradation properties make it a key functional material in the pharmaceutical and medical fields. PLGA can be completely degraded and absorbed in the human body, and by adjusting its monomer ratio, molecular weight, and polymerization process, its degradation rate can be precisely tailored to meet the requirements of various medical applications.
In pharmaceutical and medical use, PLGA demonstrates wide-ranging application value:
1.Surgical sutures: Used as absorbable sutures to avoid the need for post-operative suture removal.
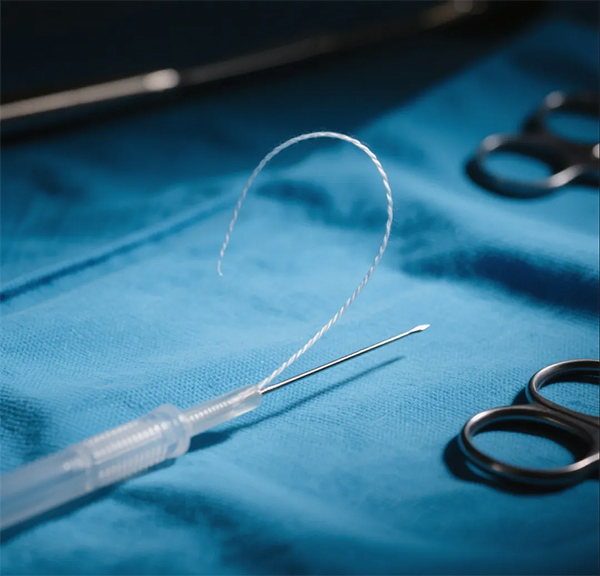
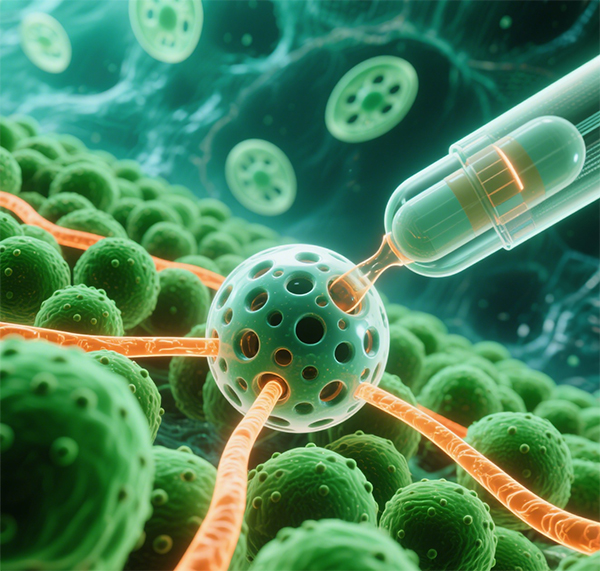
2.Drug delivery systems: Enables long-acting sustained release or targeted delivery through microspheres, nanoparticles, and other formulations.
3.Tissue engineering and orthopedic repair: Forms porous scaffolds to support cell growth and tissue regeneration; can also be used to produce bone screws, plates, and other internal fixation materials that gradually degrade after providing mechanical support.
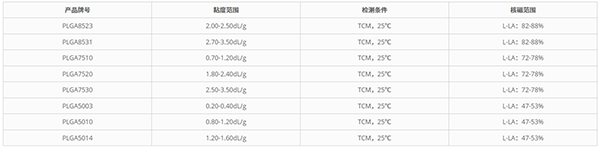
The registered PLGA raw materials include three grades: PLGA (85:15), PLGA (75:25), and PLGA (50:50). Each grade features a specific monomer ratio and intrinsic viscosity range. By precisely controlling the monomer composition, intrinsic viscosity, and polymerization technique, eSUNMed is capable of customizing the degradation cycle of the materials to meet the diverse demands of the medical industry.
The successful registration of PLGA raw materials significantly strengthens eSUNMedŌĆÖs market competitiveness. As a high-end medical material, it enhances the company’s industry standing in the biomedical materials sector, lays the groundwork for expanding into premium domestic and international markets, and provides critical support for the regulatory approval of downstream medical devices and pharmaceutical formulationsŌĆögreatly shortening product launch timelines. More importantly, this technology platform will continue to foster innovation and R&D, supporting the development of targeted drug delivery carriers, 3D printed biomaterials, and other high-value-added products.

