Background
In recent years, biodegradable polymers and additive manufacturing technologies have attracted significant attention in the field of bone tissue engineering. Polylactic acid (PLA) and its copolymers, as thermoplastic polymers with good biocompatibility and safe degradation products, have been widely applied in tissue repair. Especially when compounded with inorganic phosphate materials (such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP)), the osteogenic activity and mechanical properties can be significantly enhanced. On the other hand, selective laser sintering (SLS) technology can sinter powders layer by layer to fabricate scaffolds with complex shapes and interconnected pores. This technology has been used in the production of implants made from titanium alloys and PEKK, but both materials are difficult to degrade in the human body, posing risks of long-term complications. Therefore, combining degradable polyester materials and composites with SLS 3D printing technology to achieve âdegradation + personalized customizationâ of bone repair scaffolds has become a research hotspot. Literature reports [1â3] indicate that PLA/inorganic composite scaffolds prepared via SLS can induce osteogenic differentiation of stem cells in vitro, exhibiting excellent bioactivity and providing new ideas for bone defect repair.
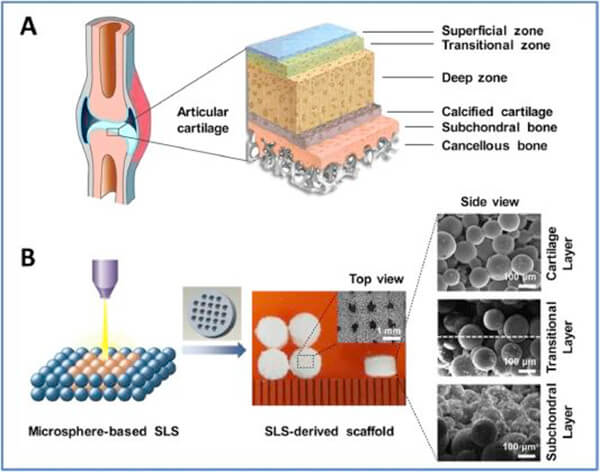
Figure 1. Natural osteochondral tissue and alveolar bone repair
What is SLS?
SLS is a powder bed fusion additive manufacturing technology that uses a high-power laser beam to selectively sinter powder material layer by layer. It does not rely on molds, and the printing path is precisely controlled by digital models, making it particularly suitable for the rapid manufacturing of customized medical devices. By controlling the laser energy and scanning algorithms, the density and microporosity of the formed parts can be regulated. Unlike traditional fused deposition modeling (FDM), SLS does not require support structures, making it suitable for fabricating complex structures with high porosity and multi-level gradients.
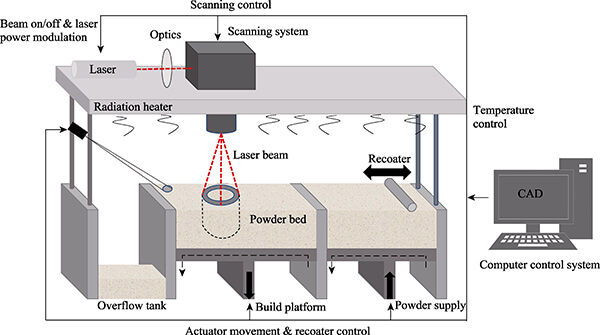
Figure 2. Schematic diagram of the SLS printing principle
Basic Characteristics and Preparation of SLS Printing Powders
SLS imposes strict requirements on powder materials, mainly including: an appropriate particle size range (generally 20â100 Îźm) with a narrow size distribution to ensure uniform powder spreading and forming accuracy; good flowability and high packing density to improve printing efficiency and part density; stable thermal performance, with suitable melting point, glass transition temperature, and thermal decomposition temperature to ensure a stable and reliable laser sintering process; good laser absorption capability; near-spherical morphology; strong storage stability; and good reusability. These characteristics collectively determine the printability of the powder in the SLS process and the final forming quality.
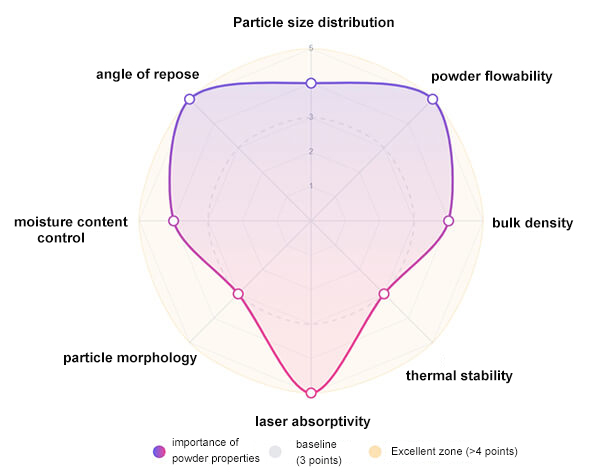
Figure 3. Key factors for SLS printing powder
The commonly used preparation techniques for polymer powders each have their own characteristics:
- Mechanical milling is economical and practical, but results in irregular powder shape and poor flowability.
- Ball milling produces powders with good regularity and flowability, but is complex and inefficient.
- Solvent precipitation obtains powders through dissolutionâprecipitation.
- Induced spheronization can prepare particles with high sphericity.
- Emulsionâsolvent evaporation is mild and allows controllable particle size, suitable for heat-sensitive substances, but has the drawbacks of complex emulsification, solvent residues, and low yield.
- Polymerization methods can synthesize microspheres with uniform particle size and high sphericity, though the particle size is often relatively small.
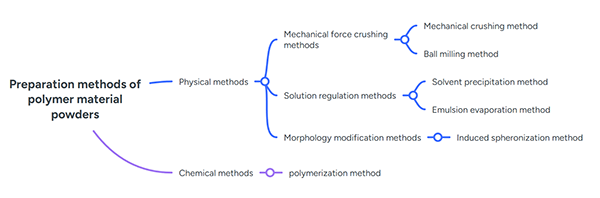
Figure 4. Common methods for preparing polymer powders
Application Research
The combination of selective laser sintering (SLS) technology and biodegradable polyesters (such as PLA, PCL, PGA, and their copolymers) has demonstrated unique and significant application value in bone repair. By precisely adjusting material composition, gradient structures, and pore characteristics, it can match the degradation period of polyesters with the bone tissue regeneration cycle, while incorporating bioactive fillers (such as HA, HAP, β-tricalcium phosphate) to enhance osteoinductive capacity. At the same time, by leveraging the process advantages of SLS, the mechanical properties and biocompatibility of scaffolds can be synergistically optimized, providing efficient, customized solutions for bone defect repair.
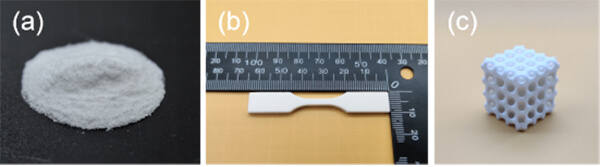
Figure 5. (a) eSUNMedâs biodegradable polyester powder for SLS 3D printing; (b) SLS 3D-printed tensile specimens; (c) SLS 3D-printed tissue scaffold.
Case 1: Multilayer PCL/HA Gradient Scaffold
Du et al. [1] constructed a bionic multilayer PCL/HA gradient scaffold via SLS for repairing rabbit knee osteochondral defects. The researchers designed a cylindrical scaffold with a diameter of 4 mm and a height of 2.8 mm, with 500 Îźm pore size and seven layers, where the HA content increased continuously from top to bottom (0â30%), and the top layer was 400 Îźm thick pure PCL. Results showed the scaffold had a porosity of approximately 60.3%, compressive modulus of 8.7 MPa, and compressive strength of 4.6 MPa. In vitro culture indicated that the multilayer scaffold supported rabbit mesenchymal stem cell adhesion well; at 7 days, the cell proliferation was significantly higher than that of pure PCL scaffolds, enhancing bioactivity. Scaffolds with 20â30% HA layers significantly improved ALP activity in stem cells, suggesting promoted osteogenic differentiation.
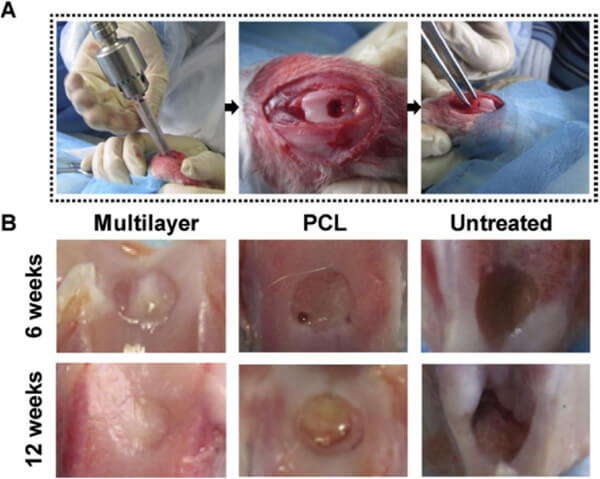
Figure 6. In vivo evaluation of SLS osteochondral scaffolds: (A) Creation of rabbit osteochondral defect models via orthopedic drilling, followed by implantation of different scaffolds; (B) Macroscopic observation at 6 and 12 weeks post-implantation showed superior cartilage repair in multilayer scaffolds compared to PCL scaffolds and untreated controls; at week 12, the multilayer group displayed cartilage-like tissue similar in appearance to surrounding natural cartilage.
Case 2: PLA/HAP Microspheres and SLS Printing
Dong et al. [2] prepared PLA/hydroxyapatite (HAP) composite microspheres using the emulsionâsolvent evaporation method. By adjusting PVA concentration, oil/water ratio, and stirring speed during preparation, regular spherical microspheres with particle sizes of approximately 40â60 Îźm were obtained. HAP was evenly dispersed within the PLA microspheres, with a porous surface structure (caused by HAP-induced pore formation). SLS was then used to print layered assemblies of the microspheres, and it was found that laser powers of 4â7 W produced good fusion qualityâthe higher the power, the better the particle bonding. Chemical analysis confirmed that laser energy input did not alter the chemical composition of the PLA/HA composite, indicating the scaffoldâs physicochemical stability.
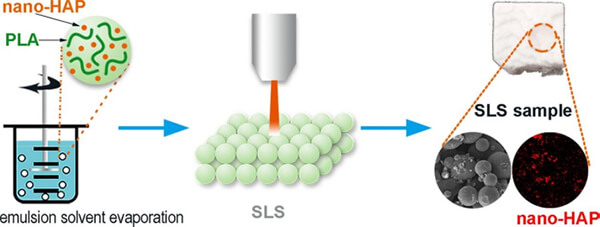
Figure 7. PLA/HAP composite microspheres prepared via emulsionâsolvent evaporation, then used in SLS laser powder bed fusion to fabricate layered parts.
Case 3: Solvent-Free PLA/CaCOâPowder and SLS
Gayer et al. [3] developed a solvent-free PLA/calcium carbonate (CaCOâ) composite powder for SLS bone scaffold printing. PLA (75 wt%) and high-purity aragonite CaCOâ (25 wt%) were co-milled into micropowder, and PLA with different intrinsic viscosities (IV) was compared. Results showed that the PLA with the lowest intrinsic viscosity (1.0 dl/g) could be milled into the smallest particles (dâ ââ50 Îźm) and had the lowest melt viscosity (~400 Pa¡s), facilitating rapid sintering. Using this PLLA-1.0/CaCOâ (75/25) powder in SLS under optimal parameters (laser power â 0.40 W) produced parts with extremely low microporosity (~2%) and a biaxial flexural strength as high as 75 MPa. The low microporosity indicated nearly complete fusion of formed particles, thus improving mechanical properties.
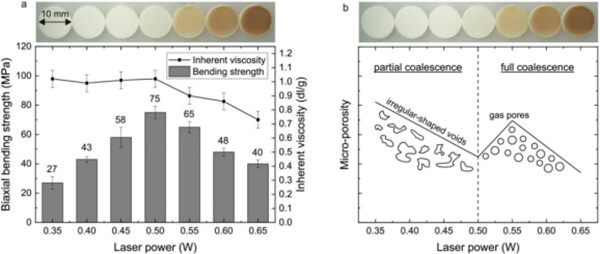
Figure 8. Circular SLS samples (diameter 10 mm, thickness 1 mm) made from PLLA-1.0-based composites: (a) Relationship between biaxial flexural strength, intrinsic viscosity, and laser power; (b) Qualitative behavior of microporosity versus laser power.
Future Outlook
Research and application of biodegradable polyester/inorganic composite powders combined with SLS additive manufacturing in bone repair scaffolds are evolving toward functionalization and high precision:
1.Material system upgrades: In the next stage, multiple bioceramics (such as calcium phosphate compounds, bioactive glassâceramics) or biomimetic polymers (collagen, gelatin, etc.) can be compounded with biodegradable polyesters to build multi-level mechanical and osteogenic gradients; simultaneously incorporating growth factors or drugs to enhance scaffold bioactivity.
2.Printing precision improvement: With advances in lasers and scanning systems, finer powders and more precise laser beam control will allow more accurate and stable scaffold structures, enabling sub-100 Îźm pores and detail features. New SLS equipment (such as dual-laser or multi-beam systems) will further improve forming efficiency and reliability.
3.Multi-functional scaffold design: Future scaffolds will not only provide mechanical support and osteoinduction but will also integrate drug or cell delivery functions, for example by loading antibacterial drugs, osteogenic growth factors, or photothermal-responsive particles inside the scaffold. In addition, smart resorbable elements can be developed to achieve dynamic mechanical response and tissue adaptation.
In summary, by integrating the latest material science and 3D printing processes, biodegradable polyesters and their inorganic composite powders combined with SLS additive manufacturing technology are expected to produce highly customizable, high-precision, and fully functional bioactive bone scaffolds, bringing more possibilities for clinical bone defect repair.
eSUNMed focuses on medical monomers, biodegradable polyesters (including PLA, PCL, PGA, and their copolymers), polyester microspheres, polyester-based composite materials, FDM 3D printing filaments, and biodegradable polyester powders and composite powders for SLS 3D printing. It also provides high-precision forming and processing services, meeting the development and application requirements of various implantable absorbable medical devices.
References
[1] Du Y, Liu H, et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials, 2017, 137: 37â48. DOI: 10.1016/j.biomaterials.2017.05.021.
[2] Dong Y, Zeng B, et al. Preparation and laser powder bed fusion of composite microspheres consisting of poly(lactic acid) and nano-hydroxyapatite. Additive Manufacturing, 2020, 34: 101305. DOI: 10.1016/j.addma.2020.101305.
[3] Gayer C, Ritter J, et al. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Materials Science and Engineering: C, 2019, 101: 660â673. DOI: 10.1016/j.msec.2019.03.101.

