eSUNMed has recently passed the ISO 13485:2016 Medical Device Quality Management System certification. The certification was issued after a comprehensive, rigorous, and meticulous on-site audit conducted by the certification bodyŌĆÖs auditors. The scope of certification covers the research, development, and sales of biomedical monomers, polymers, and microspheres used for implantable-grade devices.
This certification marks that eSUNMed has fully aligned its research and development, production, and full life-cycle quality management system of medical raw materials with international standards. It demonstrates eSUNMedŌĆÖs firm commitment to sustainably delivering stable raw materials and solutions. The certification signifies that the company now has a solid system in place to safely and reliably supply high-quality biomedical PLA and PLA microsphere materials to global customers.
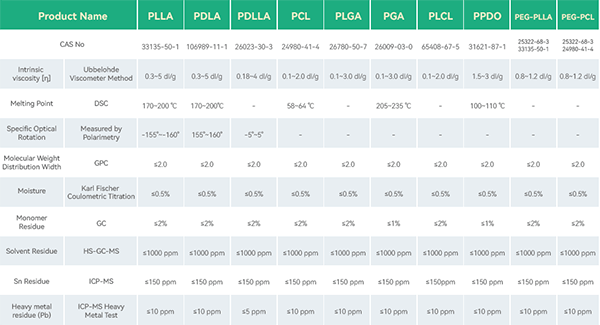
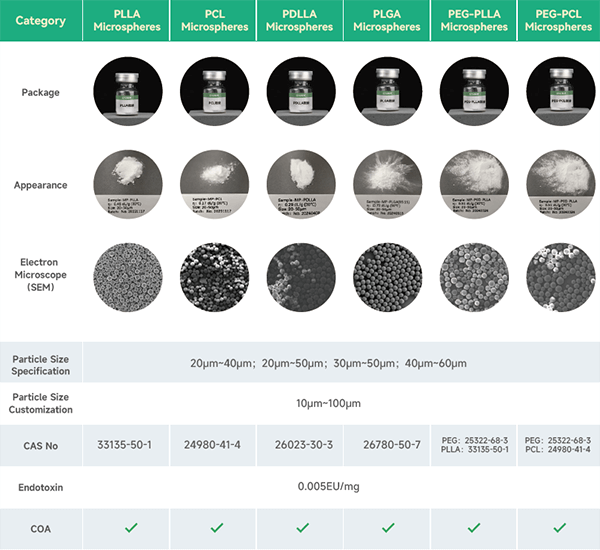
 The main products and services of eSUNMed include: supplying medical-grade raw materials such as PLA, PGA, PCL, PLGA, PLCL, PEG of various molecular weights; customized solid polymer microspheres (PLLA, PCL, PLGA, etc.) with particle sizes ranging from 10 to 100 ╬╝m; customized medical devices, medical suture implants, and drug delivery systems made of polylactic acid and polycaprolactone.
The main products and services of eSUNMed include: supplying medical-grade raw materials such as PLA, PGA, PCL, PLGA, PLCL, PEG of various molecular weights; customized solid polymer microspheres (PLLA, PCL, PLGA, etc.) with particle sizes ranging from 10 to 100 ╬╝m; customized medical devices, medical suture implants, and drug delivery systems made of polylactic acid and polycaprolactone.


As a brand dedicated to the biomedical field, eSUNMed is committed to the research, development, and industrialization of medical-grade polymer materials and microsphere products. The company is located in Longhua District, Shenzhen, with an industrialization base of more than 2,000 square meters, including nearly 400 square meters of Class 100,000 cleanroom and 100 square meters of Class 10,000 purification laboratory. The production environment strictly adheres to GMP standards for pharmaceutical excipients and sterile medical devices, providing a solid guarantee for the consistency and safety of core materials, and delivering full-chain support from raw materials to end-use applications for global customers.

