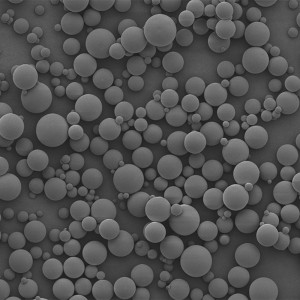
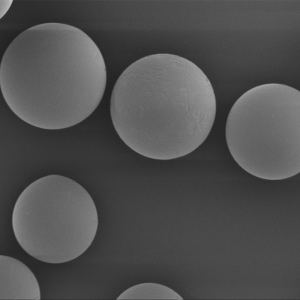
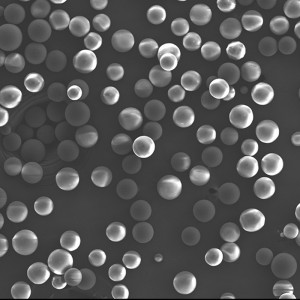
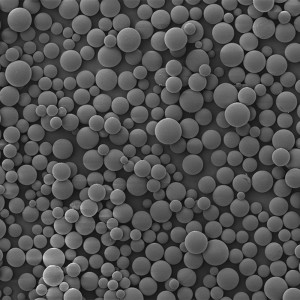
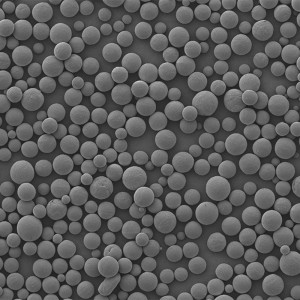
PCL Microspheres
PCL microspheres have been widely used in medical applications, especially in the field of cosmetic and plastic surgery. They can be injected subcutaneously to stimulate collagen production and improve skin elasticity, resulting in a natural-looking volumizing and rejuvenating effect.┬ĀDue to its extensive range of uses, PCL microspheres hold great promise in revolutionizing medical treatment methods, providing healthcare practitioners with more accurate, secure, and efficient treatment options while simultaneously ensuring patients a more pleasant and hassle-free experience.
Product Index:
| Item | Unit | Standard┬Ā |
Reference Standard | Method | Result |
| Appearance | / | / | / | Optical Microscopy | Spherical┬Āparticle |
| Median Size’╝łD50’╝ē | ╬╝m | 35┬▒5 | / | Laser Particle Size Analyzer | 34.35 |
| Span | / | Ōēż0.7 | / | Laser Particle Size Analyzer | 0.612 |
| Intrinsic Viscosity [╬Ę] | dL/g | 0.50┬▒0.05 | GB/T 37642-2019 | 25┬▒0.02 ┬░C, 0.01 g/mL THF Solution, 0.47 mm Inside Diameter of Automatic Ubbelohde Viscometer | 0.526 |
| Moisture | % | Ōēż 0.5 | GB/T 37642-2019 | Karl Fischer Coulometric Titration | 0.13 |
| Monomer Residue | % | Ōēż 2.0 | / | GC | 0.11 |
| Solvent Residue | ppm | Ōēż1000 | / | GC | Qualified |
| Heavy Metal Residue (Pb) | ╬╝g/g | Ōēż10’╝łExcept Sn’╝ē | YY/T 0661-2017 | Colorimetric Method of Chinese Pharmacopoeia | Qualified |
| Catalyst Residual | ╬╝g/g | SnŌēż150 | YY/T 0661-2017 | US EPA 3052:1996 & US EPA6010D2018 | 65 |
┬Ā*Produced in GMP workshop, each batch will provide the corresponding COA report.
| Model┬ĀNo. | Intrinsic┬ĀViscosity┬Ā[╬Ę]┬Ā(dL/g) | Particle size range |
| MP-PCL02A | 0.20-0.26 | 20-50 |
| MP-PCL04A | 0.35-0.43 | 20-50 |
| MP-PCL08A | 0.70-0.90 | 20-50 |
| MP-PCL12A | 1.00-1.30 | 20-50 |
| MP-PCL04B | 0.35-0.43 | 25-50 |
With complete microsphere preparation capabilities:
*PLA/PCL synthetic design and GMP production
*Excellent formulation and process development capabilities
*Excellent excipient customization ability and GMP grade excipient production
*Multifunctional synthesis laboratory
┬Ā
Medical processing service field
The processing technology of polybiotic micropowder can serve medical beauty, drug sustained release, targeted drug delivery, cosmetics and other medical related fields.