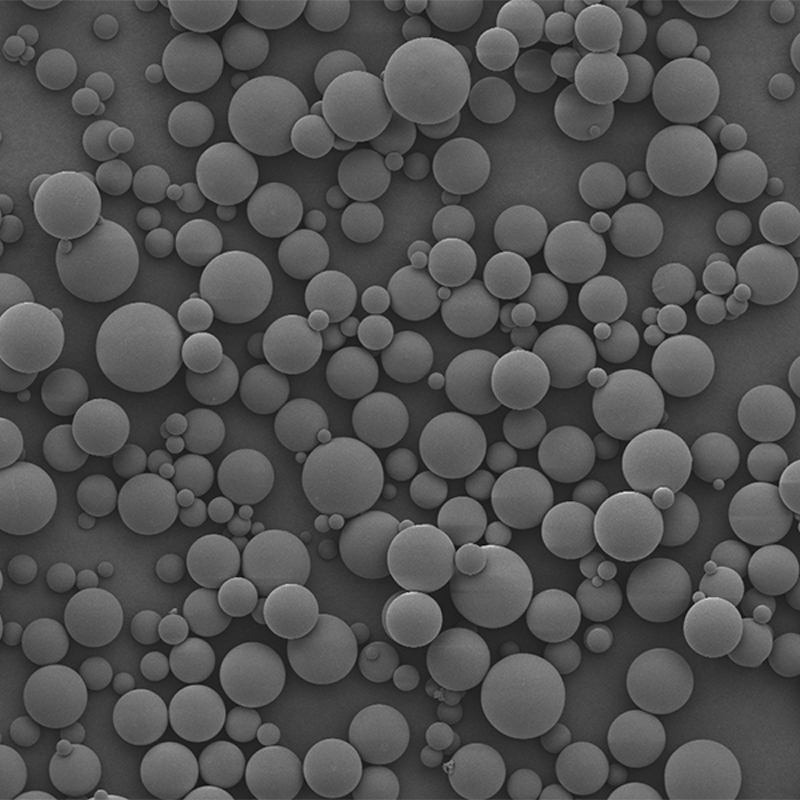
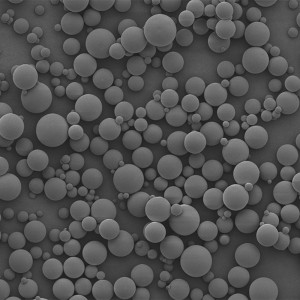
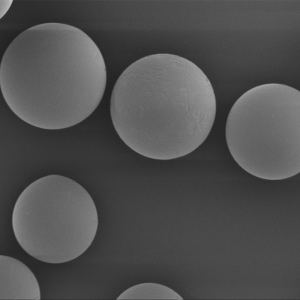
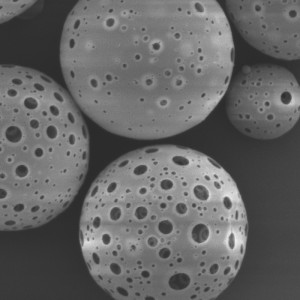
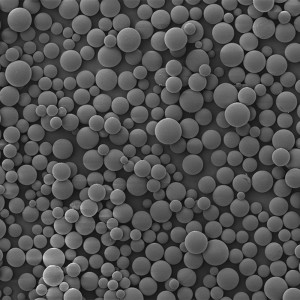
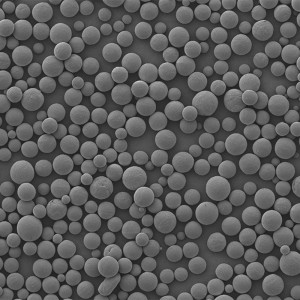
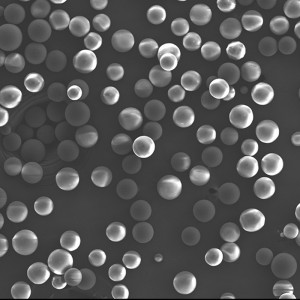
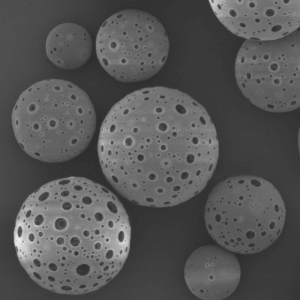
PDLLA Microspheres
PDLLA material prepared into microspheres is a subcutaneous stimulatory filler with excellent performance. It possesses similar characteristics to PLLA microspheres, but with a faster degradation rate both in vivo and in vitro. Overall, PDLLA microspheres, as a novel facial filler material, exhibit excellent biocompatibility, biodegradability, and the ability to stimulate collagen regeneration during degradation. At present, medical aesthetic filler products based on PDLLA microspheres have already been widely applied in clinical practice.
Product Index:
| Item | Unit | Standard┬Ā |
Reference Standard | Method | Result |
| Appearance | / | / | / | Optical Microscopy | Spherical┬Āparticle |
| Median Size’╝łD50’╝ē | ╬╝m | 30 ┬▒ 5 | / | Laser Particle Analyzer | 29.09 |
| Intrinsic Viscosity [╬Ę] | dL/g | 0.64┬▒0.05 | / | Refer to ASTM D 2857 in Cloroform 30Ōäā |
0.625 |
| Weight-average
Molecular Weight |
% | Ōēż0.5 | YY/T 0661-2017 | GPC | 0.45 |
| Moisture | % | Ōēż 0.5 | YY/T 0661-2017 | Karl Fischer Coulometric Titration | 0.30 |
| Monomer Residue | ppm | ICH Limit Value | YY/T 0661-2017 | GC | Qualified |
| Methanol Solvent residue | ppm | ICH Limit Value | YY/T 0661-2017 | GC | Qualified |
| Heavy Metal Residue (Pb) | ╬╝g/g | Ōēż10 | YY/T 0661-2017 | Colorimetric Method of Chinese Pharmacopoeia |
Qualified |
| Sn Residue | ╬╝g/g | Ōēż100 | YY/T 0661-2017 | ICP | 61 |
| Specific Optical Rotation | ┬░ | -10 ~ 10 | YY/T 0661-2017 | Polarimetric Test of Chinese Pharmacopoeia in Dichloromethane, 20 Ōäā |
-1.7 |
┬Ā*Produced in GMP workshop, each batch will provide the corresponding COA report.
| Model┬ĀNo. | Intrinsic┬ĀViscosity┬Ā[╬Ę]┬Ā(dL/g) | Particle size range |
| MP-PDLLA02A | 0.16-0.24 | 20-50 |
| MP-PDLLA04A | 0.32-0.48 | 20-50 |
| MP-PDLLA10A | 0.60-0.72 | 20-50 |