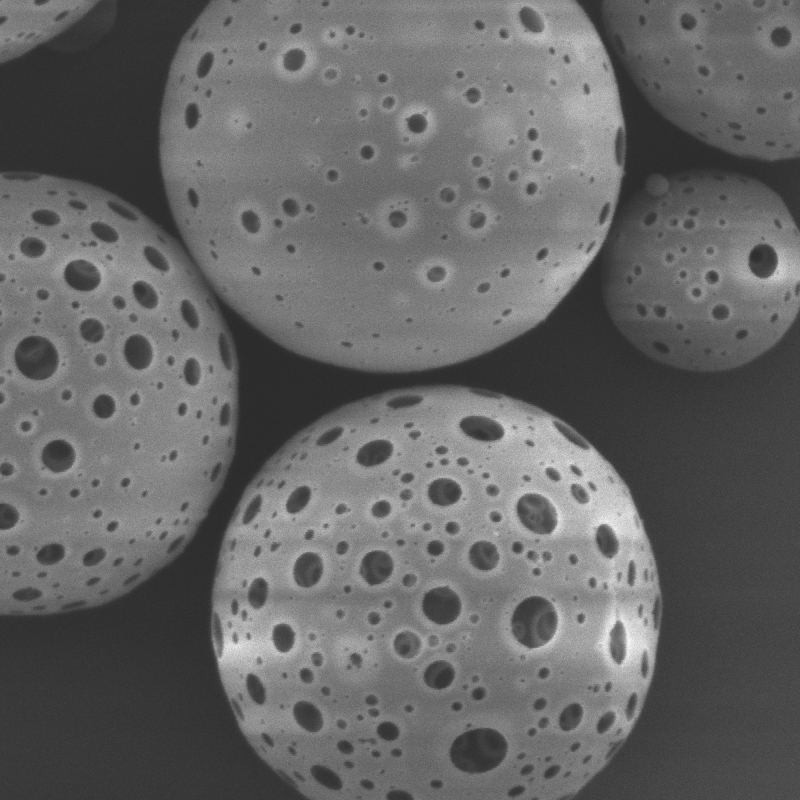
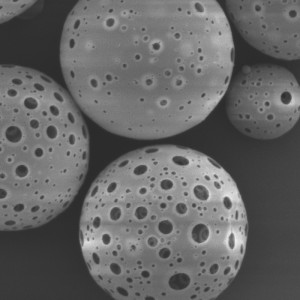
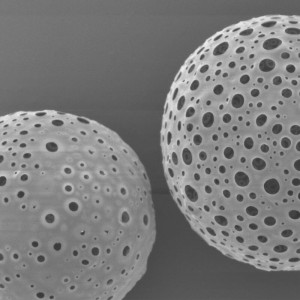
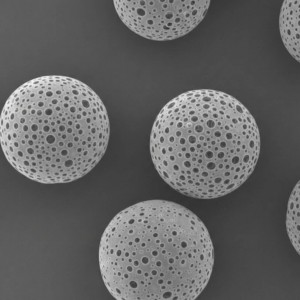
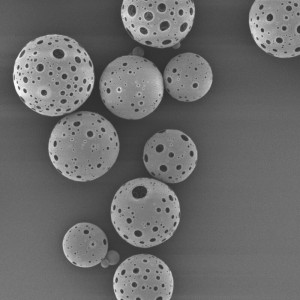
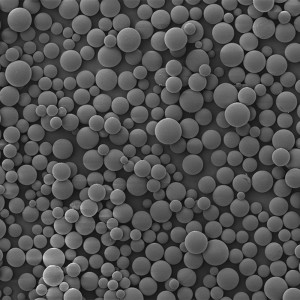
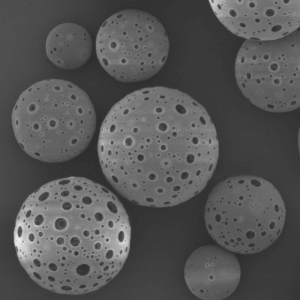
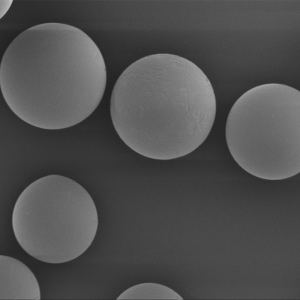
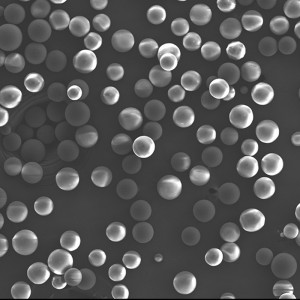
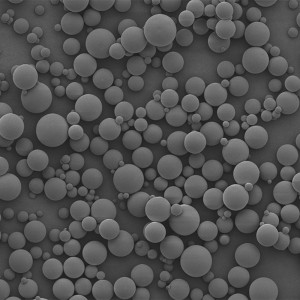
PLLA Porous Microspheres
PLLA (poly L-lactic acid) porous microspheres have been widely used in medical applications, especially in the field of cosmetic and plastic surgery. They can be injected subcutaneously to stimulate collagen production and improve skin elasticity, resulting in a natural-looking volumizing and rejuvenating effect. In addition, PLLA porous microspheres can also be used as a drug carrier to deliver therapeutic agents to target tissues or organs, such as in the treatment of liver cancer or bone defects. Due to their biocompatibility and biodegradability, PLLA porous microspheres have been shown to be safe and effective in clinical trials.
Product Index:
| Item | Unit | Standard | Reference standard | Method | Result |
|---|---|---|---|---|---|
| Appearance | / | / | / | Optical microscopy | Spherical particle |
| Median size (D50) | ╬╝m | 30-50 | / | Laser particle analyzer | 35.26 |
| Intrinsic viscosity [╬Ę] | dL/g | / | / | Refer to ASTM D 2857 in Chloroform 30 ┬░C | 0.97 |
| Moisture | % | Ōēż 0.5 | YY/T 0661-2017 | Karl Fischer coulometric titration | 0.25 |
| Monomer residue | % | Ōēż 2.0 | YY/T 0661-2017 | GC | Below the LOQ |
| Solvent residue (Total) | ppm | ICH limit value | YY/T 0661-2017 | GC | Qualified |
| PVA residue | % | < 0.2 | / | UV | Qualified |
| Sodium ion residue | ppm | / | / | IC | Qualified |
| Residual pore forming agent | ppm | / | / | IC | Qualified |
| Heavy metal residue (Pb) | ╬╝g/g | Ōēż 10 | YY/T 0661-2017 | Colorimetric method of Chinese Pharmacopoeia | Qualified |
| Sn Residue | ╬╝g/g | Ōēż 150 | YY/T 0661-2017 | ICP | Qualified |
| Specific optical rotation | ┬░ | -155 ~ -160 | YY/T 0661-2017 | Polarimetric test of Chinese Pharmacopoeia in dichloromethane, 20 ┬░C | -155.88 |
┬Ā*Produced in GMP workshop, each batch will provide the corresponding COA report.
Write your message here and send it to us